12.0 WHAT IS ENERGY?
Energy is often defined as the ability to do work. It exists in various forms which are interconvertible. When energy changes from one form to another, the total amount of energy before and after the change are always the same (Law of Conservation of Energy).
12.1 ENERGY CHANGES IN REACTIONS
Energy changes occur in chemical reactions as reactants changes to products. These changes can be observed as heat, light or sound, and are usually measured in thousands of joules. Therefore, the common unit for measuring heat energy is the kilojoule (kJ) where 1kJ = 1000J.
12.2 EXOTHERMIC AND ENDOTHERMIC REACTIONS
An exothermic reaction is one during which heat is liberated to the surroundings. Examples include:
- Reaction between an acid and a base.
- Reaction between calcium oxide and water etc.
- Combustion of fuels (e.g., burning of wood, gasoline).
- Dissolution of NaOH in water.
- Reaction of Na with water.
An endothermic reaction is one during which heat is absorbed from the surroundings. Examples are:
- Thermal decomposition of calcium trioxocarbonates(IV).
- Thermal dissociation of ammonium chloride.
- Dissolution of NH₄Cl in water.
- Melting of ice.
12.3 THERMODYNAMIC PHENOMENA
ENTHALPY – Enthalpy is defined as the heat content of a system, accounting for the energy absorbed or released during physical or chemical changes (e.g., dissolution, phase changes, or reactions). It is represented by the letter, H, then a change in the heat content of the reaction becomes ΔH (change in enthalpy).
Mathematically:
ΔH = Heat content of products – Heat content of reactants
ΔH is negative for an endothermic reaction (i.e ΔH = – x kJ mol – 1), meaning the system lost energy. and positive for an exothermic reaction (i.e ΔH = + x kJ mol – 1), indicating that the system gains energy. A calorimeter is used to determine the accurate value of ΔH.
ENTROPY – Entropy is a measure of the degree of disorder or randomness of a system. The greater the disorder, the higher the entropy i.e processes that increase disorder (like gas expansion or salt dissolution) lead to an increase in entropy. For instance:
- Mixing of Gases – When two different gases mix, they spread out and form a more disordered system. For example, when nitrogen and oxygen are mixed, the entropy increases.
- Dissolution of Salts – When a salt like NaCl dissolves in water, its orderly crystal structure breaks down into individual ions (Na⁺ and Cl⁻), leading to an increase in disorder (increased entropy).
In terms of physical state of matter, the order of increasing entropy (degree of disorder) is as follows:
Solids ˂ Liquids ˂ Gases
- Solids have the lowest entropy.
- Liquids have greater entropy than solids, but lesser than gases.
- Gases have the highest entropy.
Entropy is represented as S, while change in entropy is represented as ΔS. In a reversible reaction:
ΔS = ΔH / Temperature (T)
If reaction is endothermic, ΔS is positive (increase in entropy), while if reaction is exothermic, ΔS is negative (decrease in entropy). ΔS is measured in J K –1 mol – 1.
GIBB’S FREE ENERGY – Gibbs free energy, G, is that energy which is available for doing work. This is the driving force that brings about a chemical change. Mathematically, change in ΔG is given by ΔG = ΔH – TΔS. For all spontaneous processes, ΔG must be negative.
12.4 LAWS OF THERMODYNAMICS
THE FIRST LAW OF THERMODYNAMICS – It states that the energy can be converted from one form to another, but it cannot be created nor destroyed. Thus, during a chemical process:
Change in internal energy of a system (ΔU) = Heat absorbed by system (q) + Work done by system (w)
THE SECOND LAW OF THERMODYNAMICS – It states that a spontaneous process occurs only if there is an increase in the entropy of a system and its surroundings, i.e. the change in the total entropy of the system must be positive.
12.5 SPONTANEITY OF REACTION
A spontaneous process occurs when there is an increase in the entropy of a system and its surrounding.
CONDITIONS NECESSARY FOR SPONTANEITY OF REACTION
- ΔH must be negative (ΔH < 0).
- ΔS must be positive (ΔS > 0).
- ΔG must be negative (ΔG < 0).
Spontaneity of a reaction as determined by the sign of ΔG:
- ΔG < 0: The reaction is spontaneous.
- ΔG > 0: The reaction is non-spontaneous.
- ΔG = 0: The reaction is at equilibrium.
12.6 ENERGY PROFILE DIAGRAM
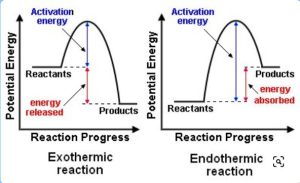
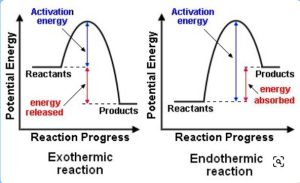
An energy profile diagram is a graphical representation of the energy changes that occur during a chemical reaction. It shows the theoretical “energy pathway” of a reaction as it progresses from reactants to products. An energy pathway is a road that a reaction travels to get from one place (the reactants) to its destination (the products). The type of energy that this pathway tracks is potential energy.
EXAMPLE – Given that in an exothermic reaction, ΔH° = −100 kJ mol – 1, ΔS° = 50 J K – 1 mol – 1 and T = 300K.
- Convert ΔS° to kJ K – 1 mol – 1.
- Calculate ΔG°.
- Determine if the reaction is spontaneous or not.
SOLUTION
- ΔS° = 50 J K – 1 mol – 1 ÷ 1000 = 0.050 kJ K – 1 mol – 1.
- ΔG° = ΔHo – TΔSo
= −100 kJ mol – 1 − (300K × 0. 050 kJ K – 1 mol – 1)
= −100 kJ mol – 1 – 15 kJ mol – 1
= −115 kJ mol – 1
- Since ΔG° is negative, the reaction is spontaneous at 300 K.
DTW Tutorials Study Resource Links;
First of All to obtain high JAMB &WAEC Scores, YOU HAVE TO Practice! Practice!! Practice!!
Use DTW JAMB & WAEC 2025 CBT Practice App!!!
– GET DTW TUTORIALS JAMB & WAEC 2025 CBT EXAM PRACTICE APP for all Subjects with over 31,000 Past Questions and Correct Solutions to Practice with offline! (Activation cost is N4000 for 1 year) Download Links Below for Mobile Phones & Laptop Computer;
DTW TUTORIALS JAMB 2025 APP For MOBILE Phone Direct Download link;
https://play.google.com/store/apps/details?id=com.iafsawii.dtw.jamb
DTW TUTORIALS JAMB 2025 APP For DESKTOP Laptop Computer Direct Download link; https://drive.google.com/file/d/1iIHBoWjEeJeCFyTO9nt-9kAveH2FqjrT/view?usp=sharing
Download Links for WAEC 2025 App;
JAMB RESOURCE LINKS BELOW;
– JAMB Past Questions Solved Playlists on Math, Phy, Chem; https://www.youtube.com/playlist?list=PLLgYU6fS5143-p4dfWIFL7keuB1SBgT2b
– THE LEKKI HEADMASTER – Summary, Questions And Answers (JAMB 2025 NOVEL); https://dtwtutorials.com/the-lekki-headmaster-jamb-2025-novel-summary-questions-and-answers-pdf-download/
– JAMB 2025 Recommended Text Books – https://dtwtutorials.com/jamb-2025-recommended-text-books-for-all-subjects/
– JAMB 2025 Syllabus all Subjects – https://dtwtutorials.com/jamb-2025-syllabus-free-download/
– JAMB 2025 Syllabus in 30 Days Timetable Challenge by DTW Tutorials for Science, Art & Commercial Subject Combinations – Cover Your JAMB Syllabus in 30 Days Challenge; https://dtwtutorials.com/jamb-2025-syllabus-in-30-days-timetable-challenge-by-dtw-tutorials-cover-your-jamb-syllabus-in-30-days-challenge/
– How to Manage Your Jamb Exam Time for High Scores; https://youtu.be/Tp4Va8haib8
– Physics Notes and Questions on All topics; https://dtwtutorials.com/category/tutorials/physics-tutorials/
– Chemistry Notes and Questions on All topics; https://dtwtutorials.com/category/tutorials/chemistry/
– How to Read, Understand and Remember Always- https://youtu.be/kL8BpRePudA
– How to Cover Your JAMB Syllabus Fast in 30 Days!!; https://youtu.be/RVgyn01Ptd0
– What to do a night before your Jamb Exam (+Exam Prayers); https://youtu.be/njbAx4Oz5Rw
– How to Manage Your Jamb Exam Time for High Scores; https://youtu.be/Tp4Va8haib8
– Overcoming Exam Fear/Anxiety– https://youtu.be/Uvf81rvd0ls
You can also join our online groups below for instant JAMB 2025 Updates;
Join DTW JAMB 2025 Intensive Tutorials Study Groups on Facebook, Telegram and WhatsApp Group;
Facebook Group – https://web.facebook.com/groups/dtwtutorialsgroup/
WhatsApp Group – https://chat.whatsapp.com/E8pprCQYtahKfpQN9UB0aU
Telegram Group – https://t.me/+AcXfhJPSIiI2ZTY0
WhatsApp Channel – https://whatsapp.com/channel/0029VaAWvTmDDmFT9o25dV3u
DTW JAMB 2025 Intensive Online Lessons/Tutorials
Online JAMB 2025 Tutorials – Your Path to Jamb Success!
Are you preparing for the JAMB 2025 Exam and aiming for excellence? Look no further than Online Jamb Tutorial by DTW Consult. We’re dedicated to helping you ace your Jamb with confidence.
- Why Choose DTW Online JAMB Intensive Tutorials?
• Engaging, Clear and Interactive Online Lectures
• Completion of JAMB Syllabus
• Weekly Quiz Assessments
• Continuous Brainstorming and Competitions
• Membership in an Active Learning Community
• Consistent Solving of JAMB Past Questions-
• Expert Jamb Instructors
• Comprehensive Study Materials - All Classes are Recorded!! In case you miss any class, and when you join us you will have access to all the previous class recorded videos!!!
• Subjects;
English
Physics
Chemistry
Biology
Math
Economics
Literature
Crs
Government
• Affordable Tuition – N7000 monthly (6pm – 10pm, Mon to Fri)
Lectures Ongoing! Register Now!!
Bank Details:
Account Name: DTW Consult
Account Number: 6414330770
Bank: Moniepoint
Amount – N7000
For easy payment and enrollment.
Proof of payment should be sent by WhatsApp.
Contact Us:
WhatsApp: 09085099582, 08038732879
Email: dtwconsultng@gmail.com
Take a step closer to your Jamb success with DTW Online Jamb 2025 Intensive Tutorials.
Let’s work together to unlock your full potential!

https://youtu.be/P7wtBH46ZMMnsive Tutorials. Let’s work together to unlock your full potential! #JambPrep #OnlineTutorial #DTWConsult #JambSuccess #jamb2025 #utme2025

No Comments