DEFINITION OF CHEMICAL COMBINATION
Chemical combination refers to the process by which two or more elements or compounds react to form new substances through the formation of chemical bonds.
- LAWS OF CHEMICAL COMBINATION
There are four laws of chemical combination which describes the general features of a chemical change. They are:
- LAW OF CONSERVATION OF MATTER – Also known as Lavoisier’s Law. It states that matter can neither be created nor destroyed during a chemical reaction but changes from one form to another.
- LAW OF DEFINITE PROPORTION – Also known as Law of Constant Composition (proposed by Proust). It states that all pure samples of a particular chemical compound contain similar elements combined in the same proportion by mass.
- LAW OF MULTIPLE PROPORTION – It states that if two elements, A and B, combine to form more than one chemical compound, then the various masses of one element, A, which combines with the fixed mass of the other element, B, are in simple multiple ratio. Examples are:
| S/N | ELEMENT A | ELEMENT B | COMPOUNDS FORMED |
| 1 | Copper | Oxygen | CuO and Cu2O |
| 2 | Iron | Oxygen | Fe2O3 and FeO |
| 3 | Iron | Chlorine | FeCl3 and FeCl2 |
- LAW OF RECIPROCAL PROPORTION – It states that the masses of several elements, A, B, C, which combine separately with a fixed mass of another element, D, are the same as, or simple multiples of, the masses in which A, B, C, themselves combine with one another.
- PARTICULATE NATURE OF MATTER
ATOMS – It is the smallest particle of an element which can take part in a chemical reaction.
MOLECULES – It is the smallest particle of a substance that can normally exist alone and still retain the chemical properties of that substance, be it an element or a compound.
IONS – An ion is any atom or group of atoms which possesses an electric charge.
- CHEMICAL SYMBOLS
Chemical symbols are used to represent elements. In 1814, Berzelius suggested a system of representing elements with symbols derived from:
- Latin names e.g Sodium, Na (Natrium); Iron, Fe (Ferrum); Copper, Cu (Cuprum); Gold, Au (Aurum) etc.
- First letter of the name of the element e.g Hydrogen (H), Oxygen (O), Sulphur (S), Fluorine (F), Carbon (C) etc.
- Initial letter and another letter from the name of the element e.g Ca for Calcium, Cl for Chlorine, Mg for Magnesium, Ne for Neon etc.
- CHEMICAL FORMULA
This is the representation of a compound or molecules of a substance by the symbols of its component elements.
Chemical formulae are majorly categorized as:
- Empirical formula – The simplest formula (shows the component elements and mole ratio).
- Molecular formula – Gives the exact number of moles of atoms of the component element in one mole of the compound.
- Structural formula – Describes the structure of a compound or molecule(s) of a substance.
Examples are O2 for oxygen gas, H2O for water, O3 for ozone, HCl for hydrochloric acid, CaCO3 for calcium trioxocarbonate (IV) etc.
- CHEMICAL EQUATION
A chemical equation is the symbolic representation of a chemical reaction, in which the reactants and the products are expressed in terms of their respective symbols and chemical formulae. The reactants are written on the left-hand side (LHS), products on the right-hand side (RHS) and an arrow leads from the reactants to the products. Example:
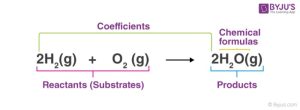
| S/N | INFORMATIONS PROVIDED BY A CHEMICAL EQUATION | INFORMATIONS NOT PROVIDED BY A CHEMICAL EQUATION |
| 1 | Reactants and products involved. | Speed of the reaction. |
| 2 | Individual elements and radicals involved. | Heat changes during reaction, (except when indicated). |
| 3 | Mental picture of the reaction. | Colours of substances involved. |
| 4 | Stoichiometry of the reaction. | |
| 5 | Direction of the reaction. | |
| 6 | State of matter of substances present. |
NB: All chemical equations must be balanced to comply with the Law of Conservation of Matter.
RELATIVE ATOMIC MASS – It is the number of times the average mass of an element is heavier than one-twelfth the mass of one atom of carbon-12.
MOLE CONCEPT – A mole of a substance is defined as the amount containing as many elementary entities as the number of atoms in exactly 12 grams of carbon-12.
1 mole = 12 grams of carbon-12 = 6.02 x 1023 atoms
Avogadro’s number = 6.02 x 1023
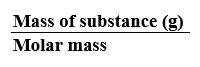
Number of moles =
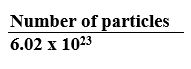
Number of moles =
STOICHIOMETRY OF REACTION
This is the quantitative relationships of the amounts of reactants to one another and to the products in a chemical reaction.
The mole ratio is the ratio in which reactants combine to form products in a chemical reaction, giving the stoichiometry of the reaction.
EXAMPLE 1 – Calculate the mass of solid product obtained when 16.8g of NaHCO3 was heated strongly until there was no further change.
SOLUTION – The equation for the reaction is:
2NaHCO3(s) → Na2CO3(s) + H2o(g) + CO2(g)
Molar mass of NaHCO3 = 23 + 12 + (16 × 3)
Molar mass of Na2CO3 = (23 × 2) + 12 + (16 × 3)
From the equation:
2 moles of NaHCO3 produces 1 mole of Na2CO3
2 × 84g of NaHCO3 produces 106g of Na2CO3
Therefore:
16.8g of NaHCO3 will produce Xg of Na2CO3
Xg of NaCO3 = (106g × 16.8g) / (2 × 84g)
= 1780.8g / 168g
= 10.6g
Mass of solid product obtained = 10.6g
EXAMPLE 2 – 20cm3 of CO was mixed and sparked with 200cm3 of air containing 21% of O2. If all the volumes are measured at s.t.p., calculate the total volume of resulting gases.
SOLUTION – In 200cm3 of air,
Volume of O2 = 21% of 200cm3
= (21 / 100) x 200cm3
= 42cm3
Volume of N2 and rare gases = 200cm3 – 42cm3
= 158cm3
The equation for the reaction is:
2CO(g) + O2(g) → 2CO2(g)
Volume ratio 2 : 1 : 2
Before sparking 20cm3 : 42cm3
Reacting volume 20cm3 : 10cm3
After sparking 32cm3 : 20cm3
Volume of resulting gases = 32cm3 of CO + 20cm3 + 158cm3 of N2 and rare gases
= 210cm3
DTW Tutorials Study Resource Links;
First of All to obtain high JAMB &WAEC Scores, YOU HAVE TO Practice! Practice!! Practice!!
Use DTW JAMB & WAEC 2025 CBT Practice App!!!
– GET DTW TUTORIALS JAMB & WAEC 2025 CBT EXAM PRACTICE APP for all Subjects with over 31,000 Past Questions and Correct Solutions to Practice with offline! (Activation cost is N4000 for 1 year) Download Links Below for Mobile Phones & Laptop Computer;
DTW TUTORIALS JAMB 2025 APP For MOBILE Phone Direct Download link;
https://play.google.com/store/apps/details?id=com.iafsawii.dtw.jamb
DTW TUTORIALS JAMB 2025 APP For DESKTOP Laptop Computer Direct Download link; https://drive.google.com/file/d/1iIHBoWjEeJeCFyTO9nt-9kAveH2FqjrT/view?usp=sharing
Download Links for WAEC 2025 App;
JAMB RESOURCE LINKS BELOW;
– JAMB Past Questions Solved Playlists on Math, Phy, Chem; https://www.youtube.com/playlist?list=PLLgYU6fS5143-p4dfWIFL7keuB1SBgT2b
– THE LEKKI HEADMASTER – Summary, Questions And Answers (JAMB 2025 NOVEL); https://dtwtutorials.com/the-lekki-headmaster-jamb-2025-novel-summary-questions-and-answers-pdf-download/
– JAMB 2025 Recommended Text Books – https://dtwtutorials.com/jamb-2025-recommended-text-books-for-all-subjects/
– JAMB 2025 Syllabus all Subjects – https://dtwtutorials.com/jamb-2025-syllabus-free-download/
– JAMB 2025 Syllabus in 30 Days Timetable Challenge by DTW Tutorials for Science, Art & Commercial Subject Combinations – Cover Your JAMB Syllabus in 30 Days Challenge; https://dtwtutorials.com/jamb-2025-syllabus-in-30-days-timetable-challenge-by-dtw-tutorials-cover-your-jamb-syllabus-in-30-days-challenge/
– How to Manage Your Jamb Exam Time for High Scores; https://youtu.be/Tp4Va8haib8
– Physics Notes and Questions on All topics; https://dtwtutorials.com/category/tutorials/physics-tutorials/
– Chemistry Notes and Questions on All topics; https://dtwtutorials.com/category/tutorials/chemistry/
– How to Read, Understand and Remember Always- https://youtu.be/kL8BpRePudA
– How to Cover Your JAMB Syllabus Fast in 30 Days!!; https://youtu.be/RVgyn01Ptd0
– What to do a night before your Jamb Exam (+Exam Prayers); https://youtu.be/njbAx4Oz5Rw
– How to Manage Your Jamb Exam Time for High Scores; https://youtu.be/Tp4Va8haib8
– Overcoming Exam Fear/Anxiety– https://youtu.be/Uvf81rvd0ls
You can also join our online groups below for instant JAMB 2025 Updates;
Join DTW JAMB 2025 Intensive Tutorials Study Groups on Facebook, Telegram and WhatsApp Group;
Facebook Group – https://web.facebook.com/groups/dtwtutorialsgroup/
WhatsApp Group – https://chat.whatsapp.com/E8pprCQYtahKfpQN9UB0aU
Telegram Group – https://t.me/+AcXfhJPSIiI2ZTY0
WhatsApp Channel – https://whatsapp.com/channel/0029VaAWvTmDDmFT9o25dV3u
DTW JAMB 2025 Intensive Online Lessons/Tutorials
Online JAMB 2025 Tutorials – Your Path to Jamb Success!
Are you preparing for the JAMB 2025 Exam and aiming for excellence? Look no further than Online Jamb Tutorial by DTW Consult. We’re dedicated to helping you ace your Jamb with confidence.
- Why Choose DTW Online JAMB Intensive Tutorials?
• Engaging, Clear and Interactive Online Lectures
• Completion of JAMB Syllabus
• Weekly Quiz Assessments
• Continuous Brainstorming and Competitions
• Membership in an Active Learning Community
• Consistent Solving of JAMB Past Questions-
• Expert Jamb Instructors
• Comprehensive Study Materials - All Classes are Recorded!! In case you miss any class, and when you join us you will have access to all the previous class recorded videos!!!
• Subjects;
English
Physics
Chemistry
Biology
Math
Economics
Literature
Crs
Government
• Affordable Tuition – N7000 monthly (6pm – 10pm, Mon to Fri)
Lectures Ongoing! Register Now!!
Bank Details:
Account Name: DTW Consult
Account Number: 6414330770
Bank: Moniepoint
Amount – N7000
For easy payment and enrollment.
Proof of payment should be sent by WhatsApp.
Contact Us:
WhatsApp: 09085099582, 08038732879
Email: dtwconsultng@gmail.com
Take a step closer to your Jamb success with DTW Online Jamb 2025 Intensive Tutorials.
Let’s work together to unlock your full potential!

https://youtu.be/P7wtBH46ZMMnsive Tutorials. Let’s work together to unlock your full potential! #JambPrep #OnlineTutorial #DTWConsult #JambSuccess #jamb2025 #utme2025

No Comments